
Research & Development

In Laboratorios Bagó we maintain and promote a strong commitment to our own research and development, materialized in the constant search for original molecules, drug associations and new pharmaceutical forms, assessment of the pharmacological profile and its pharmaceutical-analytical development.
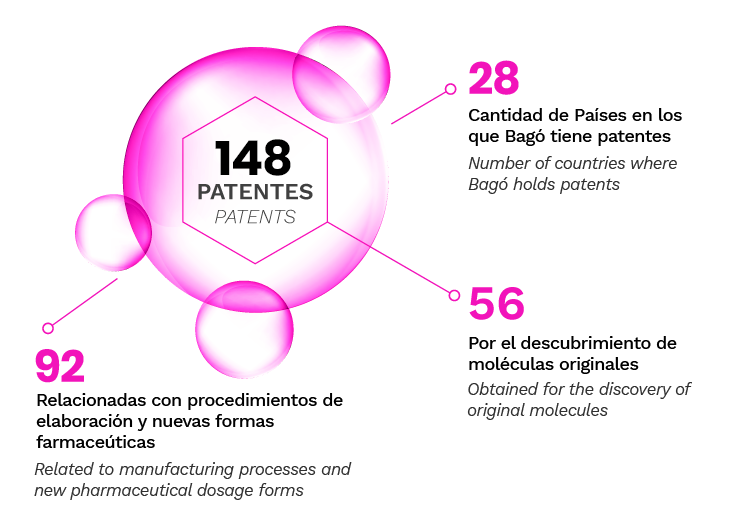
Our 148 patents in over 28 countries worldwide evidence this commitment allowing us to position our products ahead of modern therapeutics and at the level of the highest international demands and parameters.
COMMITTED TO
RESEARCH AND DEVELOPMENT
All of these achievements have been supported by the scientific rigorousness of our research teams – highly qualified professionals with a multidisciplinary background – mainly focused on the search of new therapeutic responses aimed at enhancing the quality of life, health and welfare of people.
Major R&D Achievements
-
Creation of the IBI, Instituto Bagó de Investigaciones (Bagó Research Institute), which established the scientific and medical bases oriented to the therapeutic updating of our products.
-
Development of the first injectable ampicillin in Argentina: TRIFACILINA (broad spectrum antibiotic).
-
Development and commercialization of the first association of a beta-lactam drug (ampicillin) with a beta-lactamase inhibitor (cloxacillin / dicloxacillin) in Argentina: TRIFACLOX.
-
Development and commercialization of the first association of d-propoxyphene with dipyrone: DIOXADOL/KLOSIDOL (analgesic) in Argentina and abroad.
-
First molecule researched, developed and commercialized in Latin America by an Argentine pharmaceutical company: talniflumate, non-steroidal anti-inflammatory (SOMALGEN).
-
Design and development of an exclusive drug controlled-release system, for DRYLINA 300 (Bronchodilator) products, INCORIL 90, INCORIL 120, INCORIL 180, INCORIL 240 and INCORIL 300 (Calcium Antagonist).
-
Development and commercialization of the first and unique association of amoxicillin, beta-lactam drug, with sulbactam, beta-lactamase inhibitor: TRIFAMOX IBL, in the world.
-
Development, design and commercialization of the first sublingual formulation of alprazolam anxiolytic: TRANQUINAL SUBLINGUAL.
-
Development, design and commercialization of ULCOZOL COMPRIMIDOS (TABLETS), special formulation of omeprazole (antiulcer drug).
-
The first controlled clinical trial for TRANQUINAL SUBLINGUAL as a sleep inducer (ALCMEON 47, yer XV, vol.12 n3, Oct. 2005: pp: 262-270).
-
Design and development of Omeprazole powder for oral suspension, offering sustained and rapid antacid action (DUAL ACTION). Its approval by the ANMAT enabled the rational use of omeprazole in pediatrics given its fast action and the possibility to prepare a solution to administer according to mg/kg of weight.
-
Development, design and commercialization of the first antibiotic in chewable tablets of Argentina: (amoxicillin 250 and 500 mg.). It facilitates the dosing scheme in Pediatrics, as well as in several types of adult patients, in order to achieve better adherence to the drug therapy.
-
Development, design and commercialization of the first chewable tablet of DICLOFENAC in Argentina: DIOXAFLEX. Innovative formulation (microencapsulated diclofenac) that allows obtaining adequate plasmatic levels of diclofenac for an optimum benefit-risk relation.
-
The higher efficacy and usefulness of Ulcozol Rapid to provide an enhancing therapeutic alternative in the handling of gastroesophageal reflux in pediatrics was evidenced. (Clinical trials were published in Acta Gastroenterológica Latinoamericana, presented at the US Conference on Gastroenterology “DD-Week”, and one of these clinical trials was pre-selected for an award at the Conference held by the Argentine Association of Gastroenterology 2009).
-
Laboratorios Bagó was the first pharmaceutical company in the market to associate Diclofenac and Omeprazole in a single capsule. A pharmacokinetic study proved that the new formulation of DIOXAFLEX PROTECT assures a correct absorption of both active ingredients.
-
A European patent is obtained for the formulation of the manufacturing process and procedures of Alprazolam sublingual tablet and its specific applications. It associates the use of alprazolam with “primary insomnia and anxiety-related insomnia”.
-
An Chinese patent is obtained fot the first pharmaceutical composition with anti-obesity activity comprising a premixture of pure Orlistat and preparation process, and dosage flexibility.
-
Development of an extended-release formulation of the first combination of Diclofenac (100 mg in extended-release microgranules) and Omeprazole, in the Argentine market.
Introduction in Argentina of the only Rufinamide (Saikel), highly specific antiepileptic drug for a particularly serious form of childhood epilepsy, difficult to control and with bad prognosis (known as Lennox-Gastaut Syndrome).


